Alphabetical
Avogadro's Law
[sujeto]
The relationship between a gas’s volume (V) and amount (n, in moles), which was based on the work of the Italian scientist Amedeo Avogadro. Avogadro’s Law states that at a constant pressure and temperature, a gas’s volume is directly proportional to its amount.
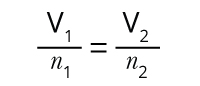
Sign in or register
For an ad-free experience and access the Visionlearning Classroom, sign in or register.
