Chemical Reactions (previous version)
by Anthony Carpi, Ph.D.
Did you know that chemical reactions happen all around us, such as when you light a match, start a car, or even take in a breath of air? To accomplish stability among atoms, sometimes electrons are taken from or shared with another atom. This need to balance electrons with protons causes chemical reactions and can create compounds.
- configuration
- the way parts are arranged
- reaction
- a chemical change when substances come into contact with each other
(A newer version of the Chemical Reactions module is available.)
The reaction of two or more elements together results in the formation of a chemical bond between atoms and the formation of a chemical compound (see our Chemical Bonding module). But why do chemicals react together? The reason has to do with the participating atoms' electron configurations (see our The Periodic Table of Elements module).
Inert gases
In the late 1890s, the Scottish chemist Sir William Ramsay discovered the elements helium, neon, argon, krypton, and xenon. These elements, along with radon, were placed in group VIIIA of the periodic table and nicknamed inert (or noble) gases because of their tendency not to react with other elements (see our Periodic Table page).
The tendency of the noble gases to not react with other elements has to do with their electron configurations. All of the noble gases have full valence shells; this configuration is a stable configuration and one that other elements try to achieve by reacting together. In other words, the reason atoms react with each other is to reach a state in which their valence shell is filled.
Metals
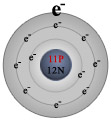
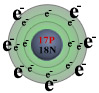
Let's look at the reaction of sodium with chlorine. In their atomic states, sodium has one valence electron and chlorine has seven.
 |
 |
| Sodium | Chlorine |
Chlorine, with seven valence electrons, needs one additional electron to complete its valence shell with eight electrons. Sodium is a little bit trickier. At first it appears that sodium needs seven additional electrons to complete its valence shell. But this would give sodium a -7 electrical charge and make it highly imbalanced in terms of the number of electrons (negative charges) relative to the number of protons (positive charges). As it turns out, it is much easier for sodium to give up its one valence electron and become a +1 ion. In doing so, the sodium atom empties its third electron shell and now the outermost shell that contains electrons, its second shell, is filled - agreeing with our earlier statement that atoms react because they are trying to fill their valence shell.
Sodium Chloride
This trait, the tendency to lose electrons when entering into chemical reactions, is common to all metals. The number of electrons metal atoms will lose (and the charge they will take on) is equal to the number of electrons in the atom's valence shell. For all of the elements in group A of the periodic table, the number of valence electrons is equal to the group number (see our Periodic Table page).
Comprehension Checkpoint
Nonmetals and metalloids
Nonmetals, by comparison, tend to gain electrons (or share them) to complete their valence shells. For all of the nonmetals, except hydrogen and helium, their valence shell is complete with eight electrons. Therefore, nonmetals gain electrons corresponding to the formula = 8 - (group #). Chlorine, in group 7, will gain 8 - 7 = 1 electron and form a -1 ion.
Hydrogen and helium only have electrons in their first electron shell. The capacity of this shell is two. Thus helium, with two electrons, already has a full valence shell and falls into the group of elements that tend not to react with others, the noble gases. Hydrogen, with one valence electron, will gain one electron when forming a negative ion. However, hydrogen and the elements on the periodic table labeled metalloids, can actually form either positive or negative ions corresponding to the number of valence electrons they have. Thus hydrogen will form a +1 ion when it loses its one electron and a -1 ion when it gains one electron.
Comprehension Checkpoint
Reaction energy
All chemical reactions are accompanied by a change in energy. Some reactions release energy to their surroundings (usually in the form of heat) and are called exothermic. For example, sodium and chlorine react so violently that flames can be seen as the exothermic reaction gives off heat. On the other hand, some reactions need to absorb heat from their surroundings to proceed. These reactions are called endothermic. A good example of an endothermic reaction is that which takes place inside of an instant "cold pack." Commercial cold packs usually consist of two compounds – urea and ammonium chloride – in separate containers within a plastic bag. When the bag is bent and the inside containers are broken, the two compounds mix together and begin to react. Because the reaction is endothermic, it absorbs heat from the surrounding environment and the bag gets cold.
Reactions that proceed immediately when two substances are mixed together (such as the reaction of sodium with chlorine or urea with ammonium chloride) are called spontaneous reactions. Not all reactions proceed spontaneously. For example, think of a match. When you strike a match you are causing a reaction between the chemicals in the match head and oxygen in the air. The match won't light spontaneously, though. You first need to input energy, which is called the activation energy of the reaction. In the case of the match, you supply activation energy in the form of heat by striking the match on the matchbook. After the activation energy is absorbed and the reaction begins, the reaction continues until you either extinguish the flame or you run out of material to react.
Table of Contents
Activate glossary term highlighting to easily identify key terms within the module. Once highlighted, you can click on these terms to view their definitions.
Activate NGSS annotations to easily identify NGSS standards within the module. Once highlighted, you can click on them to view these standards.