Though the periodic table has only 118 or so elements, there are obviously more substances in nature than 118 pure elements. This is because atoms can react with one another to form new substances called compounds (see our Chemical Reactions module). Formed when two or more atoms chemically bond together, the resulting compound is unique both chemically and physically from its parent
atoms.
Let's look at an example. The element sodium is a silver-colored metal that reacts so violently with water that flames are produced when sodium gets wet. The element chlorine is a greenish-colored gas that is so poisonous that it was used as a weapon in World War I. When chemically bonded together, these two dangerous substances form the compound sodium chloride, a compound so safe that we eat it every day - common table salt!
In 1916, the American chemist Gilbert Newton Lewis proposed that chemical bonds are formed
between atoms because electrons from the atoms interact with each other. Lewis had observed that many elements are most stable when they
contain eight electrons in their valence shell. He suggested that atoms with fewer than eight valence electrons bond together to share electrons and complete their valence shells.
While some of Lewis' predictions have since been proven incorrect (he suggested that electrons occupy cube-shaped orbitals), his work established the basis of what is known today about chemical bonding. We now know that there are two main types of chemical bonding; ionic bonding and covalent bonding.
Ionic bonding
In ionic
bonding, electrons are completely transferred from one atom to another. In the process of either losing or gaining
negatively charged electrons, the
reacting atoms form ions. The oppositely
charged ions are attracted to each other by electrostatic forces, which
are the basis of the ionic bond.
For example, during the reaction of sodium with chlorine:
| sodium (on the left) loses its one valence electron to chlorine (on the right), | 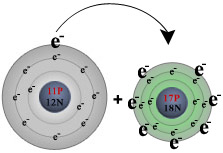 | | resulting in | ↓ | | a positively charged sodium ion (left) and a negatively charged chlorine ion (right). | 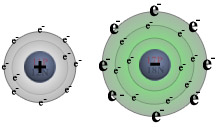 |
The reaction of sodium with chlorine
Concept simulation - Reenacts the reaction of sodium with chlorine.
Notice that when sodium loses its one valence
electron it gets smaller in size, while chlorine grows larger when it gains an
additional valence electron. This is typical of the relative sizes of ions
to atoms. Positive ions tend to be smaller than their parent atoms while
negative ions tend to be larger than their parent. After the reaction takes place, the charged Na+ and Cl- ions are held together by electrostatic forces, thus forming an ionic bond. Ionic compounds share many features in common:
- Ionic bonds form between metals and nonmetals.
- In naming simple ionic compounds, the metal is always first, the nonmetal second (e.g., sodium chloride).
- Ionic compounds dissolve easily in water and other polar solvents.
- In solution, ionic compounds easily conduct electricity.
- Ionic compounds tend to form crystalline solids with high melting temperatures.
This last feature, the fact that ionic compounds are solids, results from the intermolecular forces (forces between molecules) in ionic solids. If we consider a
solid crystal of sodium chloride, the solid is made up of many positively
charged sodium ions (pictured below as small gray spheres) and an equal number of negatively charged chlorine ions (green spheres). Due to the interaction of the charged ions,
the sodium and chlorine ions are arranged in an alternating fashion as
demonstrated in the schematic. Each sodium
ion is attracted equally to all of its neighboring chlorine ions, and likewise
for the chlorine to sodium attraction. The concept of a single molecule does not apply to ionic crystals because the solid exists as one continuous system. Ionic solids form crystals with high melting points because of the strong forces between neighboring ions.
| Sodium Chloride Crystal | NaCl Crystal Schematic |  | | Cl-1 | Na+1 | Cl-1 | Na+1 | Cl-1 | | Na+1 | Cl-1 | Na+1 | Cl-1 | Na+1 | | Cl-1 | Na+1 | Cl-1 | Na+1 | Cl-1 | | Na+1 | Cl-1 | Na+1 | Cl-1 | Na+1 |
Covalent bonding
The second major type of atomic bonding occurs
when atoms share electrons. As opposed to ionic bonding in which a
complete transfer of electrons occurs, covalent bonding occurs when two (or more) elements
share electrons. Covalent bonding occurs because the atoms in the compound
have a similar tendency for electrons (generally to gain electrons). This
most commonly occurs when two nonmetals bond together. Because both of the
nonmetals will want to gain electrons, the elements
involved will share electrons in an effort to fill their valence shells. A
good example of a covalent bond is that which occurs between two hydrogen
atoms. Atoms of hydrogen (H) have one valence electron in their first
electron shell. Since the capacity of this shell is two electrons, each
hydrogen atom will \"want\" to pick up a second electron. In an effort to
pick up a second electron, hydrogen atoms will react with nearby hydrogen (H) atoms to form
the compound H2. Because the hydrogen compound is a combination
of equally matched atoms, the atoms will share each other\'s single electron,
forming one covalent bond. In this way, both atoms share the
stability of a full valence shell.
Covalent bonding between hydrogen atoms
Concept simulation - Recreates covalent bonding between hydrogen atoms.
Unlike ionic compounds, covalent molecules exist as true molecules. Because electrons are shared in covalent molecules, no full ionic charges are formed. Thus covalent molecules are not strongly attracted to one another. As a result, covalent molecules move about freely and tend to exist as liquids or gases at room temperature.
Multiple Bonds: For every pair of electrons shared between two atoms, a
single covalent bond is formed. Some atoms can share multiple pairs of
electrons, forming multiple covalent bonds. For example, oxygen (which has
six valence electrons) needs two electrons to complete its valence shell.
When two oxygen atoms form the compound O2, they share two pairs of
electrons, forming two covalent bonds.
Lewis Dot Structures: Lewis dot structures are a shorthand to represent the valence electrons of an atom. The structures are written as the element symbol surrounded by dots that represent the valence electrons. The Lewis structures for the elements in the first two periods of the periodic table are shown below.
Lewis structures can also be used to show bonding between atoms. The bonding electrons are placed between the atoms and can be represented by a pair of dots or a dash (each dash represents one pair of electrons, or one bond). Lewis structures for H2 and O2 are shown below.
Polar and nonpolar covalent bonding
There are, in fact, two subtypes of covalent bonds. The H2 molecule is a good example of the first type of covalent bond, the
nonpolar bond. Because both atoms in the H2 molecule have an equal attraction (or affinity) for electrons, the bonding electrons are equally shared by the two atoms, and a nonpolar covalent bond is
formed. Whenever two atoms of the same element bond together, a
nonpolar bond is formed.
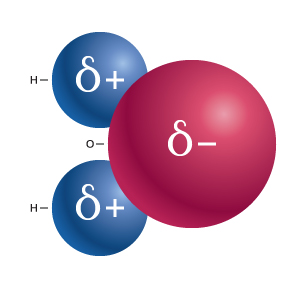 |
A water molecule: H2O. |
A polar bond is formed when electrons are unequally shared between two atoms. Polar covalent bonding occurs because one atom has a stronger affinity for electrons than the other (yet not enough to pull the electrons away completely and form an ion). In a polar covalent bond, the bonding electrons will spend a greater amount of time around the atom that has the stronger affinity for electrons. A good example of a polar covalent bond is the hydrogen-oxygen bond in the water molecule.
Water molecules contain two hydrogen atoms (pictured in red) bonded to one oxygen atom (blue). Oxygen, with six valence electrons, needs two additional electrons to complete its valence shell. Each hydrogen contains one electron. Thus oxygen shares the electrons from two hydrogen atoms to complete its own valence shell, and in return shares two of its own electrons with each hydrogen, completing the H valence shells.
Polar covalent bonding simulated in water
The primary difference between the H-O bond in water and the H-H bond is the degree of electron sharing. The large oxygen atom has a stronger affinity for electrons than the small hydrogen atoms. Because oxygen has a stronger pull on the bonding electrons, it preoccupies their time, and this leads to unequal sharing and the formation of a polar covalent bond.
The dipole
Because the valence electrons in the water molecule spend more time around the oxygen atom than the hydrogen atoms, the oxygen end of the molecule develops a partial negative charge (because of the negative charge on the electrons). For the same reason, the hydrogen end of the molecule develops a partial positive charge. Ions are not formed; however, the molecule develops a partial electrical charge across it called a dipole. The water dipole is represented by the arrow in the pop-up animation (above) in which the head of the arrow points toward the electron dense (negative) end of the dipole and the cross resides near the electron poor (positive) end of the molecule.
Related Modules
• Chemical Reactions
• Chemical Equations
| 
