Teoría Atómica II: Iones, neutrones, isótopos y teoría cuántica
¿Sabías que la energía no se libera en un flujo continuo, sino en “paquetes”? Este descubrimiento, conocido como teoría cuántica, cambió la forma en que entendemos las propiedades básicas del átomo. En el siglo XX se produjeron muchos otros avances en la teoría atómica, incluido el descubrimiento del neutrón, que hizo posible la bomba atómica.
Las primeras ideas acerca de la materia en un nivel atómico se formaron en el transcurso de muchos siglos. Comenzando con los Griegos antiguos y moviéndose por el principio del siglo XIX, el estudio se desarrolla de manera relativamente lenta. (Puede leer mas acerca de esto en nuestros módulos Las Primeras ideas acerca de la Materia: Desde Demócrito hasta Dalton and Teoría Atómica I: Los primeros días.) A pesar del ritmo lento, es crucial entender que el proceso era un proceso metódico ya que científicos se basaban en ideas anteriores. Esta progresión gradual y lógica en donde la estructura atómica se desarrollo de ser una idea simple y filosófica hasta un mundo sofisticado de la partícula Higgs Bosón descubierta en la primera parte del siglo XXI, representa a un maravilloso ejemplo de la evolución de una idea científica, y la aplicación del proceso científico. De hecho, uno podría argumentar que la historia, la lucha y el acontecimiento que se ha hecho por medio del desarrollo del entendimiento de la materia en un nivel atómico es la típica historia del método científico.
Evidencia que llevó al cambio
La historia de la teoría atómica se encuentra primero con evidencia científica y reproducible a finales del siglo XVIII. Químicos franceses Antoine Lavoisier y Joseph Proust, con su Ley de Conservación de Masa en el año 1789 y la Ley de Proporciones Definidas en 1799, respectivamente, cada uno dejó las bases para el trabajo del inglés John Dalton acerca de la Ley de Múltiples Proporcionas (Dalton, 1803). Dado que muchos siglos han pasado desde las primeras ideas del átomo y el trabajo de Dalton, seria justo decir que la evolución de Teoría Atómica ha sido una gradual, con los adelantos en el campo siendo fijos en vez de espectacular. Pero todo eso estaba por cambiar y muy dramáticamente.
El periodo mas intenso del progreso se llevó a cabo entre finales del siglo XIX y principios del siglo XX y se basaba altamente en el trabajo del físico danés llamado Niels Bohr. Como tantos antes de el, Borh se basó en el trabajo de sus predecesores y para Borh, parte de esas bases habían sido hechas por Ernest Rutherford.
.Basándose en una serie de experimentos, Rutherford propuso el modelo planetario del átomo en el cual electrones giran alrededor de un núcleo denso (vea Teoría Atómica I: Los Primeros días). Mientras que el modelo de Rutherford explicaba muchas observaciones con precisión, igual tenia errores.
Bohr avanza las ideas de Rutherford
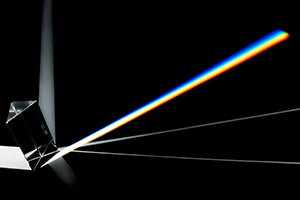
El modelo planetario del átomo de Rutherford se basaba en la física clásica – un sistema que trata con partículas físicas, fuerza y momento. Desafortunadamente, el mismo sistema predice que los electrones girando en la manera que Rutherford describía que perderían energía, liberaría radiación y ultimadamente, chocarían en el núcleo y destruiria el átomo. Sin embargo, por su mayor parte, los átomos son estables, durando literalmente miles de millones de años. Además, la radiación predicha por el modelo de Rutherford hubiera sido un espectro continuo de cada color – esencialmente luz blanca que cuando es pasada por medio de una prisma mostraría todos los colores de una arcoíris (Figura 1).
Pero cuando gases puros de diferentes elementos son estimulados por electricidad, como hubieran estado en el recién descubierto tubo de descarga eléctrica, emiten radiación en distintas frecuencias. En otras palabras, diferentes elementos no emiten luz blanca, emiten luz de diferentes colores y cuando la luz pasa a través de una prisma no produce una arcoíris continua de colores, pero el patrón de líneas coloradas, ahora se refieren como la línea espectro (Figura 2). Claramente, el modelo de Rutherford no cabía con todas las observaciones y Bohr se puso a hablar de estas inconsistencias.
Figura 2: El espectro de luz visible se desplaza arriba de la línea espectro durante tres meses – el hidrogeno, neón y el hierro están abajo.
image ©Neon spectrum: Deo FaventeEn el año 1911, Niels Bohr (Figura 3) había recién completado su doctorado en la física en la Universidad de Copenhague y fue invitado para continuar su trabajo en la Universidad de Manchester en Inglaterra por Rutherford. Rutherford había avanzado significativamente la teoría atómica con su experimento revolucionario, pero el genio de Bohr estaba avanzando aún mas el modelo de Rutherford.
Figura 3: Niels Bohr
Fue Bohr a quien se le ocurrió la idea original de un modelo planetario del átomo, pero fue el quien tomo el concepto fundamental y le aplicó nuevas ideas acerca de la teoría cuántica. Este salto fue necesario para explicar la nueva evidencia que retó el modelo viejo y para subsecuentemente formular un modelo mejor.
Animación Interactiva: Bohr’s Atom
Punto de Comprensión
Max Planck y la Teoría Cuántica
Es fácil pensar que la luz y otras formas de energía, como continua. Subale a una lámpara y la lámpara se hace gradualmente mas brillante. Sin embargo, a finales del siglo XIX, los físicos comenzaban a sospechar que esto no era verdadero. Modelos clásicos físicos no pudieron predecir con exactitud la radiación de cuerpo negro; en otras palabras no predijeron exactamente la energía liberada por un objeto cuando se calienta
El físico alemán Max Planck resolvió este problema en el año 1903, al proponer que la energía de radiación de cuerpo negro puede ser cuantizada (en otras palabras que solo podía ser liberada o absorbida en “paquetes” específicos que estaban asociados con frecuencias especificas. Esto resolvió el problema del cuerpo negro y fue consistente con los datos experimentales observados. Así nació la mecánica cuántica.
A pesar de los avances que el y otros hicieron utilizando esta idea, curiosamente, Planck permaneció escéptico de energía cuantizada por muchos años. Insistió que los cálculos que había hecho y las conclusiones que había alcanzado eran un truco matemático sofisticado y ultimadamente el modelo clásico prevalecerá. Después de todo, habían existido aproximadamente 200 años y había pasado por escrutinio bien intenso.
Avanza la Teoría Cuántica
En el año 1905, Albert Einstein publicó una serie de artículos proponiendo que la luz también exhibe comportamiento cuántico (Einstein, 1905). Algunas veces descrito como el Annus Mirabilis (año milagroso) de Einstein, los artículos combinados con el trabajo de Planck, permitieron que Bohr combinara la naturaleza del átomo con la física para marcar el comienzo del entendimiento de la teoría atómica.
En el año 1913, basándose en las teorías de Planck y Einstein de la cuantización, Bohr propuso que un electrón fuese cuantizado – no podía existir en cualquier lado alrededor del átomo (como fue sugerido en el modelo de Rutherford) pero en vez de esto, podía ser encontrado en posiciones especificas con energías especificas. El electrón podía transicionar a diferentes posiciones, pero solamente en pasos discretos y definidos. No podía girar en cualquier ubicacion alrededor del núcleo del átomo pero en vez de esto estaba restringido a especificas áreas de espacio – tal como planetas en nuestro sistema solar están restringidas a caminos específicos.
Siendo negativamente cargados, los electrones se atraen a protones específicos en el núcleo del átomo y normalmente ocuparía la ruta dentro del átomo que esta mas cerca del núcleo si esa ruta esta disponible. Este estado, la cual tiene energía potencial baja es llamado estado fundamental. Al exponer los electrones a una fuente externa de energía como una descarga eléctrica, es posible promover los electrones de su estado fundamental a otras posiciones que tienen energías potenciales mas altas llamadas estados excitados. Estos electrones “excitados” rápidamente regresan a posiciones de energía baja (para poder volver a obtener la estabilidad asociada con energías bajas) y al hacer esto liberan energía en frecuencias especificas que corresponden a la diferencia de energías entre orbitales de electrones o capas (vea simulación de comportamiento cuántico). Las ecuaciones matemáticas de Bohr predijeron que los electrones no chocarían con el núcleo en una manera que la física clásica (y el modelo de Rutherford) había predicho. Esto fue otra realización importante que hizo el salto de una paradigma (física clásica) a una nueva (física cuántica).
Animación Interactiva: Atomic and ionic structure of the first 12 elements
El descubrimiento de Bohr que la teoría cuántica de Planck puede ser aplicada al modelo clásico de Rutherford del átomo y podría explicar las deficiencias observadas en el modelo original, es otro bello ejemplo de cómo la teoría científica utiliza evidencia anterior junto con nuevas observaciones experimentales para adaptar, desarrollar y cambiar modelos y entendimiento con el paso de tiempo. La ciencia es usualmente avanzada por científicos contemporáneos basándose en el trabajo de sus predecesores y como dijo Isaac Newton en su carta para Robert Hooke (ambos científicos prominentes en sus épocas) en el año 1676 por “pararse en los hombros de gigantes”. El trabajo de Bohr se baso en las teorías de aquellos antes de el y le extendieron para explicar los espectros de línea observados experimentalmente de átomos en una prueba de matemáticas que tenia bastante sentido.
Punto de Comprensión
La formación de iones
Mientras que el trabajo de Bohr parecía que explicaba el fenómeno curioso de espectros de líneas, un número de líneas habían sido observadas en la espectro de hidrógeno que no cabía dentro de la teoría de Bohr, pero Bohr ofreció una explicación rápidamente. Sugirió que las líneas en el espectro de hidrógeno que no podían ser contabilizadas eran causadas no por átomos de hidrogeno, si no que por una especie completamente diferente. Entonces, ¿que eran estas especies, y como llegaron a hacer?
Casi 30 años antes de que Bohr publicara su famosa trilogía de artículos en la revista científica Philosophical Magazine and Journal of Science en el año 1913 la idea de que partículas tienen la habilidad de llevar un tipo de carga había sido establecida por el científico Sueco Svante Arrhenius y el Inglés Michael Faraday. Estas partículas cargadas fueron llamadas iones.
Los átomos son eléctricamente neutros, lo que significa que el número de protones positivos en cualquier dado átomo es igual al numero de electrones negativos en el mismo. Ya que la carga positiva es exactamente cancelada por la negativa, el átomo no tiene ninguna carga eléctrica. Sin embargo, así como es posible estimular un electrón para que se mude a una orbita mas alta como había mostrado el trabajo de Bohr, también es posible dar a un electrón suficiente energía para sobrepasar la atracción del núcleo completamente y removerlo del átomo del todo. Esto tiene un efecto de desequilibrar la carga eléctrica y resulta por la formación de una especie con una carga positiva – llamado catión. Por ejemplo, el átomo de sodio puede perder un electrón para formar un catión de sodio positivamente cargado (Ecuación 1); la energía asociada con la expulsión del primero electrón de cualquier átomo llamado la primera energía de ionización.
Na(s) → Na+(s) + e-
Cationes que son formados por la expulsión de un electrón puede ser ionizado adicionalmente al perder mas electrones, y en el proceso formando otro ion, esta vez con una carga de 2+. La energía requerida por la expulsión del segundo electrón es conocido como segunda energía de ionización. A pesar de que raramente ocurre con átomos mas grandes (o con el ejemplo de sodio anterior), es retóricamente posible remover todos los electrones de cualquier átomo, llevando al tercero, cuarto, quinto, etc. la energía de ionización para átomos con grandes números de electrones.
Figura 4: Utilizando el elemento hidrógeno, ejemplos de catión y anión.
image ©JkwchuiUna vez que el electrón (o electrones) ha sido sacado del átomo de esta manera, puede ser aceptado por otros átomos, y tal, los electrones pueden ser transferidos de un átomo a otro. Así como liberar electrones desbalancea la carga, aceptar electrones también causa que los átomos se desbalanceen en carga, (mas electrones que protones) y al especie se llama anión. (Un catión y un anión de hidrógeno se muestran en la Figura 4.) Por ejemplo, un átomo neutral de cloro, con números iguales de protones y electrones, pueden aceptar un electrón de una fuente externa para formar un anión de cloruro cargado negativamente (ecuación 2).
Cl(g) + e- → Cl-(g)</sub)
Iones tienen propiedades muy diferentes en comparación a sus átomos originales, y esta transferencia de electrones de un átomo a otro es un ejemplo de que como un pequeño cambio en la estructura de un átomo puede hacer una gran diferencia en su comportamiento y la naturaleza de las partículas. Por ejemplo, el metal de sodio es inestable, reaccionando violentamente con agua y se corroe instantáneamente con el aire. Por ende, cuando viene en contacto con metal de sodio seria extremadamente peligroso. Similarmente el cloruro existe como gas bajo condiciones ambientales y es altamente venenoso, cicatrizando los pulmones de cualquiera que lo respira (de hecho, el gas de cloro fue utilizado como una arma química durante la Primera Guerra Mundial). Sin embargo, cuando las dos sustancias reaccionan una con otra, el sodio pierde un electrón formando un catión en el proceso, y el cloro acepta el mismo electrón para formar un anión. Los dos resultantes iones que se enlazan después se enlazan y juntos crean una sustancia muy común – la sal de mesa, la cual no es ni reactiva ni venenosa.
Punto de Comprensión
Bohr relaciona las observaciones inesperadas a iones
En el trabajo de Bohr, la ionización de un elemento particular, el helio, se comprobo ser la clave para descifrar la explicación de las líneas inesperadas que observó en el espectro de hidrogeno. Cuando un átomo de helio, la cual tiene dos electrones pierde un electrón para formar un ion de helio, He+, la estructura electrónica es igual a la del hidrógeno atómico, ya que ambas especies tienen solamente un electrón – el ion de helio se dice ser isoeléctrico con el átomo de hidrogeno. Sin embargo, el ion de hielo posee un núcleo con el doble de la carga del átomo de hidrógeno (dos protones en vez de una). Bohr se dio cuenta de esto y sugirió que la atracción mas grande entre el electrón y el núcleo He resultaba en líneas espectros que anteriormente no se podían explicar – la carga del núcleo afecta la energía asociada con transferencias de electrones entre los orbitales. La teoría de Bohr fue comprobada ser correcta cuando espectros fueron generados utilizando helio que había sido purgado de hidrógeno.
Descubrimiento de una tercera partícula atómica: El neutrón
Al final del siglo XIX, dos diferentes partículas se sabia que existían en el átomo, y ambos poseían una carga eléctrica – el pequeño y negativamente cargado electrón y el mas grande y positivamente cargado protón. Sin embargo, a principios del siglo XX, evidencia comenzó a acumularse que esto no era todo lo que había en el átomo. Específicamente, la masa de protones y electrones en el átomo no aparentaron ser lo suficiente para justificar la masa de todo el átomo, y ciertos tipos de decaimiento nuclear sugirieron que algo mas debe suceder en el núcleo. En el año 1932, James Chadwick, un físico Británico que había estudiado con Ernest Rutherford y que en ese entonces trabajaba con el mismo, se propuso resolver el problema. Rutherford desde el año 1920 se propuso la idea de una partícula atómica neutral que tenia masa, pero nunca llego a agilizar la búsqueda de esta partícula misteriosa.
En el año 1932, Chadwick desarrolló mas un experimento que había sido llevado a cabo por Frederic Joliot-Curie con Irene Joliot-Curie. Encontraron que al utilizar polonio como fuente de partículas alfa, pueden usar berilio para emitir radiación que puede ser utilizada para eliminar protones de un pedazo de parafina. Los Joliot-Curies propusieron que esta radiación era radiación gama, un paquete de energía sin masa verdadera. Como un investigador de renombre de radiación gama y del núcleo del átomo, Chadwick realizó lo que otros no podían, que los protones eran demasiado grandes para ser sacados de parafina por rayos de gama sin masa. Al medir mas cuidadosamente el impacto de la partícula misteriosa en la parafina y al combinar esto con otras medidas, Chadwick concluyó que las partículas siendo emitidas no eran radiación gama, pero una partícula relativamente pesada que no tenia carga - una partícula llamada neutrón.
Figura 5: Modelo artístico de un átomo mostrando el núcleo, con protones y neutrones orbitando electrones.
image ©VisionlearningChadwick escribió un artículo acerca de su descubrimiento llamado “La Posible existencia de un Neutron,” y fue publicado en la revista Nature (Chadwick, 1932). En 1935, fue otorgado el Premio Nobel en la Física por su descubrimiento. Los Joliot-Curies tampoco fueron sin reconocimiento. Su trabajo en la radioactividad y los isotopos radioactivos les obtuvo el Premio Nobel en la Química, también en el año 1935.
El descubrimiento de Chadwick marcó el génesis de reacciones nucleares inducidas en donde el neutrón es acelerado y lanzado al núcleo de los elementos, generando cantidades masivas de energía (el neutrón puede hacer esto fácilmente ya que es neutral y no es repelado del núcleo en la misma manera que partículas cargadas podrían serlo). Estas reacciones tuvieron un impacto masivo en el mundo entero ya que dieron luz a pensamientos de la bomba atómica y energía nuclear.
Punto de Comprensión
Otros cambios en la estructura de átomos: Isotopos
El neutrón también explica porque la existencia de átomos del mismo elemento que tienen diferentes masas atómicas. Los isotopos son átomos del mismo elemento (i.e. tienen el mismo número de protones) pero la diferencia en el número de neutrones que poseen. Como resultado, diferentes isotopos tienen propiedades químicas similares, pero sus masas y en algunos casos su comportamiento físico son diferentes. Los isotopos se diferencian por su masa atómica, la cual se puede indicar escribiendo el símbolo del elemento, seguido por un guión y después la masa, o mas comúnmente, escribiendo la masa sobrescrita antes del símbolo elemental. Por ejemplo carbono-12 (C-12, o 12C) y carbono-14 (C-14, or 14C) son ambos isotopos de carbón que ocurren naturalmente. El carbono-12 es un isotopo estable que cuenta como 99% del carbón que ocurre naturalmente. El carbono-14 es un isotopo radioactivo que cuenta como solamente 1 x 10-10 % del carbón que ocurre naturalmente, ya que decae a nitrógeno con un periodo de semidesintegración de aproximadamente 5,730 años, y puede ser utilizado para fijar la fecha de algunos objetos que contienen carbono. (Tres isotopos de carbono se miran en la Figura 6.)
Figura 6: Isotopos de carbón. Cada uno con el mismo número de protones, pero con diferentes números de neutrones.
A menudo ocurren naturalmente mas de un isotopo en cualquier elemento individual. Como resultado, las masas atómicas que se dan en una tabla periódica moderna son las masas ponderadas de todos los isotopos conocidos de cada elemento. Por ejemplo, el cloro que ocurre naturalmente tiene dos isotopos principales, uno con 18 neutrones (masa de 35) y uno con 20 neutrones (masa de 37). El isotopo mas liviano 35Cl, cuenta como casi 76% de su abundancia natural, mientras que el isotopo 37Cl cuenta como solamente 24%. Por ende, la media ponderada es la masa promedio de átomos que ocurren naturalmente ponderados por su abundancia relativa, o 35.45.
Punto de Comprensión
El trabajo de Bohr proveyó un puente entre varias ideas disparatas que quizás nunca estuvieron enlazadas sin su intervención. El cambió el paradigma de aplicar física clásica (de partícula) al modelo del átomo a pensar acerca de la aplicación de la teoría cuántica y ondas – un desarrollo crucial en el gran esquema de teoría atómica y uno que fundó las bases para futuros científicos.
Habiendo haber llegado desde su trabajo inicial, hasta la teoría atómica por medio de la crucial primera parte del siglo XX, aún tenemos mucho por explorar en la historia del átomo. Los avances descritos en este modulo se basan en el trabajo de Rutherford, modificado por las ideas de Planck, y juntado por el genio de Bohr. Aún así, hubiera por lo menos otra década por pasar antes de que el trabajo de Pauli, Heisenberg y ultimadamente Schrödinger llevara al desarrollo completo de la mecánica cuántica moderna que completamente describe el átomo como lo conocemos hoy en día.
Este módulo es una versión actualizada de nuestro contenido previo, para ver el modulo anterior por favor visite: link.