Alphabetical

ideal gas
[noun]
A theoretical gas which behaves as predicted by the ideal gas equation. The molecules in an ideal gas are assumed to have no volume, and to experience no intermolecular forces of attraction or repulsion. The ideal gas equation uses the gas constant, R, and describes the relationship between a gas’s pressure (P), volume (V), amount (n, in moles), and absolute temperature (T, in Kelvins)
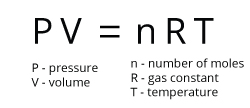
Sign in or register
For an ad-free experience and access the Visionlearning Classroom, sign in or register.
