Ácidos y Bases I: Definiciones, pH y neutralización
¿Sabías que no hace falta ser un científico profesional para utilizar la química ácido-base? Cuando comes demasiados alimentos picantes, es el ácido el que provoca la “quemazón” en el estómago. Y cuando buscas alivio con medicamentos o bicarbonato de sodio en agua, tu objetivo es provocar una reacción química llamada neutralización.
This is an updated version of the module Acids and Bases.
If you’ve ever cared for a pet fish, you know that you can’t just add plain tap water to the tank. If you do, you’ll throw off the chemistry of the environment the fish needs to survive, and the fish may die. One important aspect of a fish’s environment is the acidity of the solution it’s swimming in.

Acidity is the quality that gives liquids such as vinegar and lemon juice their lip-puckering taste. In fact, the term acid comes from the Latin term acere, which means "sour”. The chemical opposites of acids are bases, compounds that often feel soapy or slippery and lend bitterness to foods such as walnuts and broccoli.
While there are many slightly different definitions of acids and bases, for purposes of this discussion of the fundamentals of acid/base chemistry, we’ll introduce you to two definitions: the Arrhenius definition and the Brønsted-Lowry definition. In addition, we will introduce you to the pH scale and to the process of neutralizing acids and bases, which will keep your fish healthy.
Las primeras descripciones químicas de ácidos y bases
People have known for centuries that there were substances like lemons and vinegar that shared that characteristic of being acere (sour). It was the Irish writer and amateur chemist Robert Boyle in the mid-1660s, who first labeled substances as either acids or alkalies (which we now call bases), according to the following characteristics:
Acids taste sour, corrode metals, and change litmus (a dye that changes color based on acidity) red.
Bases taste bitter, feel slippery, and change litmus blue.
Boyle also observed that some of the characteristics of acids can be counteracted by adding base, and vice versa. While he and others tried to explain why acids and bases behave the way they do, the first reasonable definition of acids and bases would not be proposed until 200 years later.
La definición de Arrhenius primero explica la química ácido/base
In the late 1800s, the Swedish scientist Svante Arrhenius proposed that water can dissolve many compounds by separating them into their individual ions. Arrhenius suggested that acids are compounds that contain hydrogen and can dissolve in water to release hydrogen ions into solution. For example, hydrochloric acid (HCl) dissolves in water as follows:
(The "aq" in parenthesis stands for "aqueous," meaning that the ions are dissolved in a water-based solution.)
In contrast, Arrhenius defined bases as substances that dissolve in water to release hydroxide ions (OH-) into solution. For example, a typical base according to the Arrhenius definition is sodium hydroxide (NaOH):
The Arrhenius definition of acids and bases explains a number of things. Arrhenius’ definition explains why all acids have similar properties to each other and, conversely, why all bases are similar. Acids all release H+ into solution while bases all release OH-. The Arrhenius definition also explains Boyle's observation that acids and bases counteract each other. This idea, that a basic solution becomes less basic when mixed with an acid, and vice versa, is called neutralization - a concept we’ll discuss further in this lesson.
Though Arrhenius helped explain the fundamentals of acid/base chemistry, his ideas have limits. For example, the Arrhenius definition does not explain why some substances, such as common baking soda (NaHCO3), can neutralize acids (thus acting like bases) even though they do not contain hydroxide ions.
Punto de Comprensión
La definición de Brønsted-Lowry ofrece una imagen más completa de los ácidos y las bases.
In 1923, the Danish scientist Johannes Brønsted and the Englishman Thomas Lowry published independent yet similar papers that refined Arrhenius' theory. In Brønsted's words, "... acids and bases are substances that are capable of splitting off or taking up hydrogen ions, respectively." The Brønsted-Lowry definition broadened the Arrhenius concept of acids and bases.
Like Arrhenius, Brønsted-Lowry defines acids as any substances that can donate hydrogen ions to a solution (under the Brønsted definition, acids are often referred to as proton donors because an H+ ion - hydrogen minus its electron - consists of simply one proton).
The Brønsted definition of bases is, however, quite different from the Arrhenius definition. Brønsted defined a base as any substance that can accept a hydrogen ion. In essence, bases are the opposite of acids. NaOH, as we saw above, would still be considered a base because once dissolved in water, the OH- can accept an H+ from an acid to form water.
The Brønsted-Lowry definition also explains why substances that do not contain OH- can also act like bases. Antacid tablets are a good example of this. If you look at the ingredients on a package of an antacid, you’ll likely see calcium carbonate, or CaCO3, among them. To understand why this compound works as an antacid, take a moment to think about your favorite pizza (which, if it has tomato sauce on it, is rather acidic).

When you eat food (or even begin thinking about eating), your stomach produces hydrochloric acid, HCl, a strong acid that breaks down food to help in digestion. In the acid-water mixture in your stomach, the HCl dissociates, meaning it splits up into its constituent ions, in this case hydrogen and chloride:
Those H+ ions make the solution in your stomach acidic. But too much of it, and you’ll feel it as indigestion. So, you take an antacid, which dissociates to become calcium and carbonate ions:
The CO32- ion acts as an effective Brønsted-Lowry base, able to mop up the excess H+ ions floating around in your stomach. Ah, relief.
In this example, the carbonic acid formed (H2CO3) undergoes rapid decomposition to water and gaseous carbon dioxide:
While this reaction reduces the acidity in your stomach, the gaseous carbon dioxide produced can accumulate in your stomach and eventually cause you to burp. So, all in all, best to avoid overeating foods like pizza.
Under both the Arrhenius and Brønsted-Lowry definitions, both acids and bases are related to the concentration of hydrogen ions present. Acids increase the concentration of hydrogen ions, while bases decrease the concentration of hydrogen ions (by accepting them). The acidity or basicity of something, therefore, can be measured by its hydrogen ion concentration.
Punto de Comprensión
pH: La medida de la concentración de H+
In 1909, the Danish biochemist Sören Sörensen invented the pH scale for measuring acidity. Sörensen was studying the activity of enzymes—proteins that catalyze chemical reactions in living things—and noticed that the acidity of the solution affected the ability of the enzymes to catalyze reactions. At the time, there was no good way to articulate how acidic one compound was compared to another. Frustrated by the lack of a measurement system, Sörensen invented the pH scale. While the exact meaning of pH remains debatable, some believe it stands for “power of hydrogen.”
No matter what the letters stand for, pH is a way of measuring the concentration of H+ ions in solution that are available to participate in a reaction. (To review concentration, see the section on molarity in our module Solutions, Solubility, and Colligative Properties. The pH scale is a logarithmic scale, meaning that for each increment of 1 on the scale, say the change from a pH of 2 to a pH of 3, the concentration of H ions changes by a factor of ten. A pH of 2 has 1 x 10-2 mol H+/L and a pH of 3 has 1 x 10-3 mol H+/L.
Note that the exponent on the concentration is a negative number. For that reason, mathematically, pH is actually the inverse log of the concentration of H+ in the solution:
Don't be intimidated by the math! Even if you're not familiar with logarithms, this one is still fairly easy to understand. You can think of a logarithm as the exponent on the 10 in the [H+]. For example, a solution with [H+] = 1 x 10-7 moles/liter has a pH equal to 7. (The pH is positive because we’re taking the negative log, -(-7), and the two minus signs become a positive.
Let’s think about water. At room temperature, pure water has a pH of 7, which means that [H+] (the concentration of hydrogen ions) is 1x10-7, or 0.0000001. A pH of 7 is considered neutral, meaning that [H+] and [OH-] are equal.
A concentration of 1x10-7, or 0.0000001 mol/L might seem pretty small, but changes in concentration on either side of neutral can make a big difference in the chemistry. When [H+] is greater than 10-7 (for example, if it equals 1 x 10-6), the solution is basic. If [H+] is less than 10-7 (for example, if it equals 1 x 10-8), the solution is acidic.
If the exponents and concentrations are confusing, think of it this way: Remember that when we’re dealing with a negative exponent, we’re talking about the number of zeros you put between the decimal and the 1. The more zeros, smaller the number gets, and the less H+ you have in solution.
For example: 0.0000001 (1x10-7) is ten times larger than 0.00000001 (1x10-8). Since pH is the measure of [H+], 1x10-8 represents an [H+] that is ten times smaller than 1x10-7.
Now, compare the exponents on those two numbers: -7 and -8. If we take the negative sign away, we have the value of the pH. 1x10-7 Is a pH of 7, and 1x10-8 is a pH of 8. So, contrary to what you might expect, a higher pH number represents a smaller [H+].
The presence of H+ ions is what causes acidity, and conversely, the lack of H+ (or presence of OH-) is what causes basicity. So as [H+] gets smaller (and the pH value gets higher), a solution becomes less acidic, and more basic. Since 7 is a neutral pH, a solution with a pH greater than 7 is basic, because it has an [H+] smaller than 1x10-7. By the same token, a solution with a pH of less than 7 is acidic, and has an [H+] larger than 1x10-7.
For example:
For an [H+] of 1 x 10-3, the pH is 3 and the solution is acidic.
For an [H+] of 1 x 10-11, the pH is 11 and the solution is basic.
The pH scale ranges from 0 to 14. Substances with a pH from 0 to 7 are considered acidic. Substances with a pH greater than 7 and up to 14 are considered basic. Right in the middle, at pH = 7, are neutral substances, for example, pure water. The relationship between [H+] and pH is shown in the table below alongside some common examples of acids and bases in everyday life.
| [H+] | pH | Example | |
|---|---|---|---|
| Acids | 1 X 100 | 0 | HCl |
| 1 x 10-1 | 1 | Stomach acid | |
| 1 x 10-2 | 2 | Lemon juice | |
| 1 x 10-3 | 3 | Vinegar | |
| 1 x 10-4 | 4 | Soda | |
| 1 x 10-5 | 5 | Rainwater | |
| 1 x 10-6 | 6 | Milk | |
| Neutral | 1 x 10-7 | 7 | Pure water |
| Bases | 1 x 10-8 | 8 | Egg whites |
| 1 x 10-9 | 9 | Baking soda | |
| 1 x 10-10 | 10 | Tums® antacid | |
| 1 x 10-11 | 11 | Ammonia | |
| 1 x 10-12 | 12 | Mineral lime - Ca(OH)2 | |
| 1 x 10-13 | 13 | Drano® | |
| 1 x 10-14 | 14 | NaOH |
Punto de Comprensión
Neutralización de ácidos y bases
Let’s go back to our earlier example of overindulging on pizza. The pH table shows that the HCl in your stomach has a pH of 1, very acidic. It’s the amount of acid in your stomach that makes you feel the “burn” in “heartburn.” The antacid tablets, remember, contain the base calcium carbonate, CaCO3, which accounts for their pH of 10. When we add the CaCO3 base to the HCl mixture, the resulting CO3-2 ion is able to absorb H+ ions, bringing the mixture closer to a neutral pH of 7. This is a type of neutralization reaction in which a base is added to an acid to raise the pH (or vice versa).
We can also carry out a neutralization reaction in the laboratory. For example, we can add NaOH to a picture of HCl to neutralize the acid. In this case the neutralization reaction involves an H+ from the acid and an OH- from the base. (This is an instance in which the Arrhenius definition comes in handy). The two ions combine to form a water molecule, taking both the acidic and basic ions out of solution.
The dissociation reactions are:
And
As you can see from the equations, the acidic HCl releases H+ into solution and the NaOH base releases OH-. If we mix the acid and base together, the H+ ions combine with the OH- ions to make water, H2O:
When each H+ from the acid has been paired with an OH- and neutralized, the solution will have a pH of 7.
Titulación: uso de la neutralización para determinar el pH de una solución
Neutralization can also be used to determine the pH of a solution. For example, let’s say you need to neutralize acidic runoff from a mining operation. You know that the particular mining process you’re looking at produces HCl, but you don’t know the concentration of HCl in the runoff. You can determine the HCl concentration by neutralizing a sample of the runoff with a solution of base that you do know the concentration of and keeping track of how much of the base solution was required. From there, you can calculate HCl concentration in the runoff.
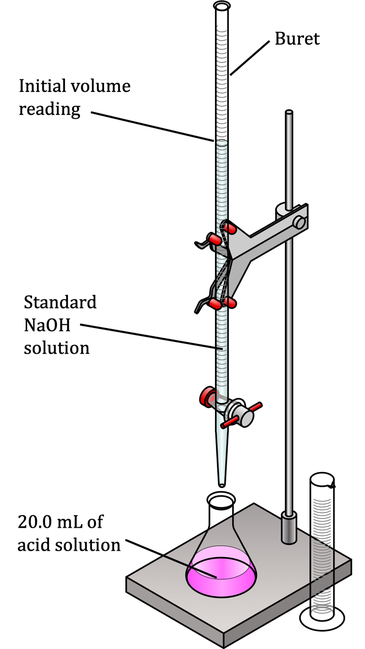
The process of determining pH by neutralizing a solution of unknown concentration with one of known concentration is called titration. In the lab, titration involves a setup with specific equipment that maintains the accuracy of the process, because you need to know the starting volume of acid and the amount of base used.
As seen in Figure X, 20 mL of the runoff solution containing HCl has been measured out and placed in a flask. A burette (essentially a graduated cylinder with a stopcock at the bottom that allows you to add base in measured increments) is filled with 1M NaOH and positioned so that it is easy to add small quantities of base to the acid. (If you need to review molarity, see our module Solutions, Solubility, and Colligative Properties.
The HCl solution also contains a small amount of a molecule called a pH indicator. An indicator is a substance that, when dissolved in water, will change color at a specific pH, indicating that the solution in the flask has reached that pH value. In this case, you would use an indicator that changes color at pH 7, because you want to know when the acid solution has been completely neutralized.
To do the titration, you note the starting volume of 1M NaOH. Let’s say it’s 500 mL. You slowly add base just until the point where the solution in the flask changes color, and then stop. Then note volume of base after addition to the acid, let’s say it’s 460 mL.
500 mL starting – 460 mL ending = 40 mL 1M NaOH added to the acid in the flask
Because we know the concentration of the NaOH, we can determine how many moles of it are in our 40 mL
Solving for x:
x = 0.40 mol NaOH added
We know that in water, NaOH releases a single OH-, and HCl releases a single H+, which means that for every mole of HCl, one mole of NaOH is required to neutralize it. Since we used 0.40 mol of NaOH in the titration, there must be 0.40 mol of HCl in the flask.
To figure out the concentration of the HCl, we then take the number of moles and divide it by the volume:
Now we can see that the concentration of HCl in the runoff solution is 2 mol/L. If the mine produces 1000 L of runoff, we know that runoff will contain 2 mol HCl/L, and therefore will require 2000 mol of base to be neutralized.
Let’s return to the real-life example of our fish tank. Over time, plants, rocks, and the fish themselves will alter the pH of the water. Most fish can adjust to a pH that changes slowly over time, but are very sensitive to sudden changes in pH. So when it’s time to clean the tank and add new water, we want to add water that is near the pH of what the fish have been swimming in.
If you’ve ever performed this task, you did a titration without even knowing it! In this case, you may not be trying to reach a pH of 7, but instead, you want to make a batch of tap water that matches the existing pH. To help with that effort, you can use a pH testing kit. Instead of a proper titration setup, you’ll have a bucket of water and an aquarium pH testing kit. The kit contains a pH test solution, a color reference card, an acidic solution and a basic solution.
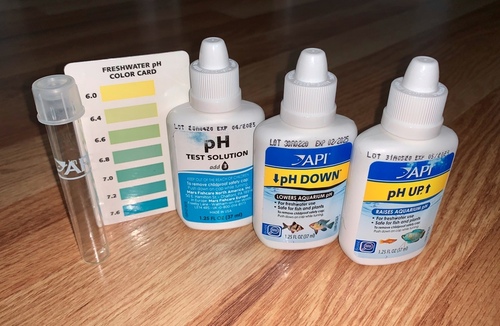
First, you test the pH of a small sample of the water that was in the tank by adding a few drops of the pH testing solution, which contains a pH indicator. You can then compare the color of the indicator to the color reference card. The different colors on the card indicate color changes associated with very subtle differences in pH around the value at which the indicator changes color.
Let’s say the pH is 5.5, which is a bit acidic. Now that you know the pH of the water in the tank, you can fill a bucket with the volume of new tap water you want to add, and use the test solution again to determine the pH. If the pH is a neutral 7 like pure water, it’s too basic, and you’ll need to titrate it with a bit of the acidic solution, testing small samples of the water as you add acid, until the color indicates the pH you want. Once you’ve gotten the pH of the new water where you want it, you can add it to the tank knowing that you won’t give your fish a pH shock.